Dr.-Ing. Martin Sch?ler, Head of Engineering & Design at Fette Compacting, and his colleagues have developed the Containment Guard to predict the retention performance of containment tabletting systems.


Dr. Ing. Martin Sch?ler is an expert in the field of containment and widely recognized throughout the industry.
In the new containment manual from Fette Compacting Sch?ler summarizes the results of his work and offers an easy-to-understand introduction to the subject. The following book excerpt deals with the classification of measured data and shows the physical challenges that containment has to face.
"Active pharmaceutical ingredients (API), are often divided into so-called bands. The Occupational Exposure Bands (OEB) are intended to enable the hazards of active ingredients to be roughly assigned. This procedure is initiated by researching pharmaceutical companies that already have to assign the hazard level for new active ingredients without however having completed the studies on the effect of the substance (animal or human studies).
Furthermore, this classification allows pharmaceutical companies to define specific minimum protective measures for classes of active ingredients. There are many different class definitions that as a rule are company-specific. All class systems refer to the grouping of the active pharmaceutical ingredient (API), irrespective of the total quantity handled or the dilution of the active ingredient. The OEB classification however also has its disadvantage in that the grouping of a pure active ingredient into a class is often mistaken by system manufacturers with the description of system features, that actually refer to the mixtures of active ingredients and excipients.
Apart from assigning the limit values to the substance systems, it is also hard to imagine the magnitude of the concentrations. This is mainly because there are virtually no comparative figures in real life, where a substance is available in quantities within the μg range. In order to clarify the concentrations in the containment area, analogies are therefore often used.
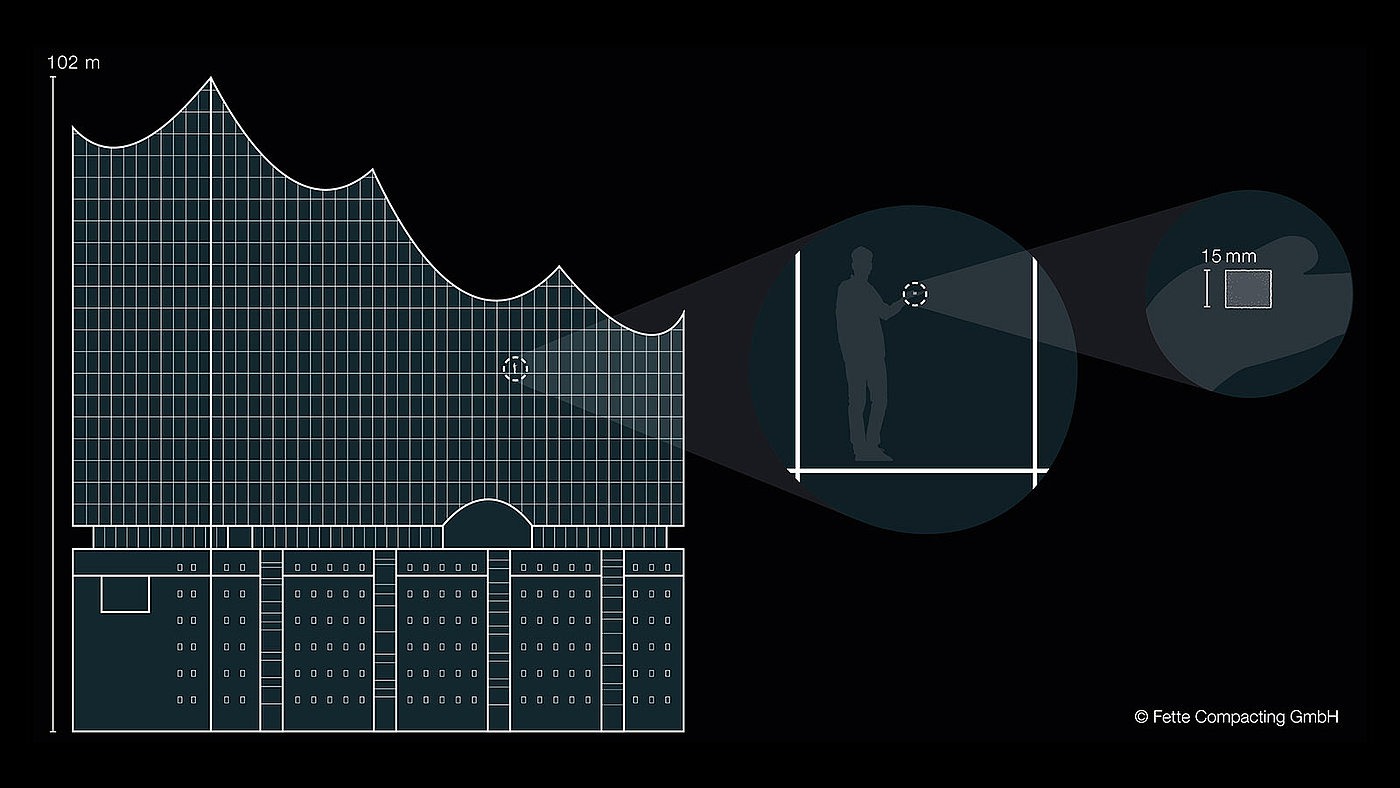
The illustration of ratios is hereby based on the use of familiar reference elements such as for example the Elbphilharmonie in Hamburg and an ordinary sugar cube. One of these sugar cubes weighs 3 g. If the sugar cube is distributed evenly in the Hamburger Elbphilharmonie (length and height above 100 m), this then gives an indoor air concentration of approx. 5.5 μg/m3. Even though the comparison may seem extreme, these are concentrations that must be adhered to on a daily basis in the pharmaceutical industry.

A single sugar cube distributed evenly in the Elbphilharmonie Concert Hall in Hamburg: In pharmaceutical production such low indoor air concentrations must be maintained day in and day out.
Pharmaceutical companies are obliged to define limit values for active pharmaceutical ingredient used during production and to provide evidence of how these limit values have been determined. In addition to this, there are also publicly accessible lists for wellknown substances, that document the limit values and that are available in order to comply with occupational safety and health. For new active pharmaceutical ingredient and those not yet fully explored, the data available is however often incomplete, which is why the initial classification of an active pharmaceutical ingredient may be much higher (safer) and then drops over time as more detailed results of the effect become available."
Please do not hesitate to contact us if you have any queries.