A breath of fresh air blows through the laboratory and the halls in Schwarzenbek in northern Germany: At Fette Compacting headquarters, pharmacists and machine experts are continuing to advance our development towards becoming an integrated process partner. This change opens up far-reaching advantages for manufacturers.
Time is a critical factor in the pharmaceutical and nutraceutical market. Efficiency and flexibility therefore play a central role from the development phase onward. Pharmaceutical companies in particular are under enormous pressure to bring new drugs to market faster without compromising on quality and safety. This is precisely where Fette Compacting’s new strategy comes in.
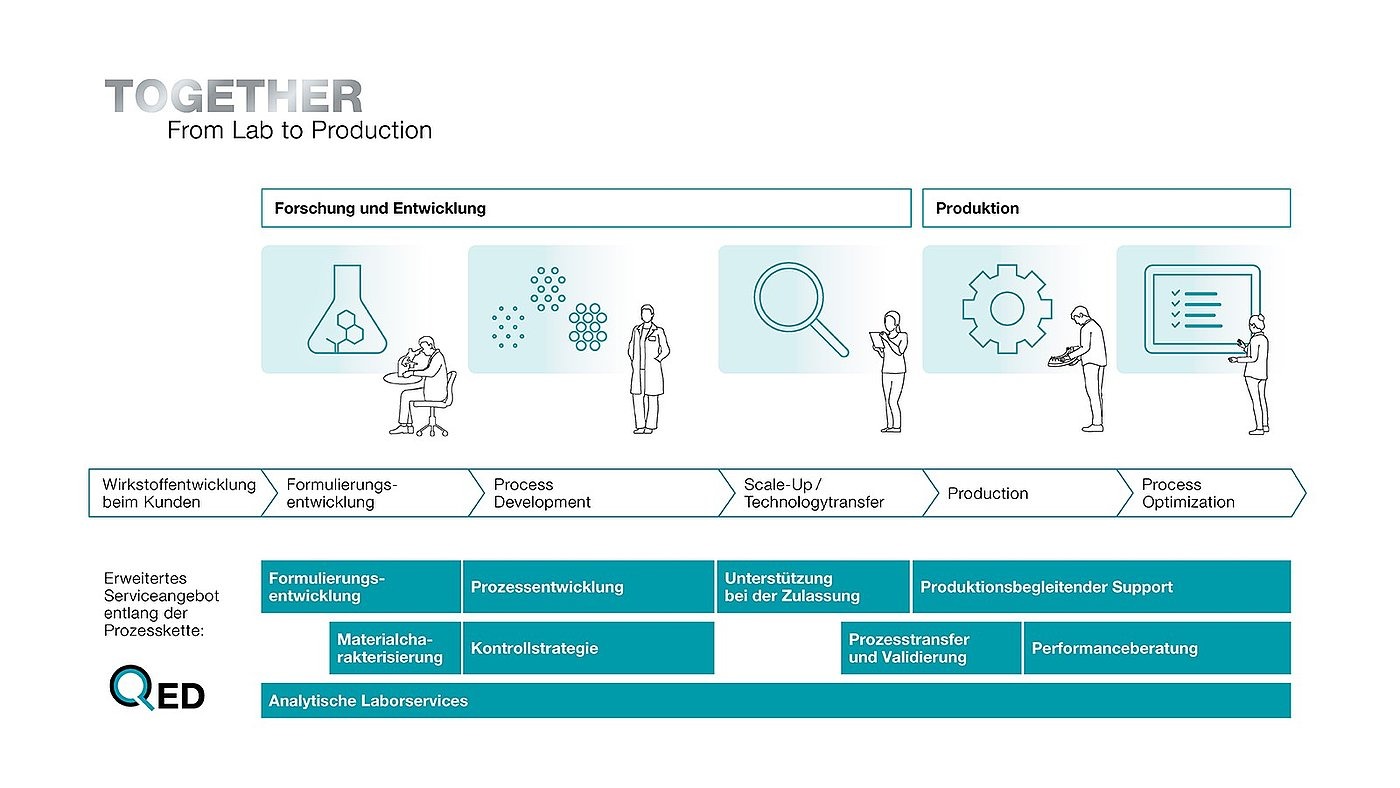
“We acknowledge that it is no longer enough to supply highly efficient machines and process equipment. Our aim is to use our decades of expertise and intensive, partnership-based exchanges with our customers to accompany the entire process from the initial idea to the marketable product – ‘Together – from lab to production,’” explains Joachim Dittrich, CEO of Fette Compacting. In addition to efficiency gains, this approach also promises tangible added value for customers: Pharmaceutical manufacturers can optimize their processes, react more quickly to market changes, and accelerate the launch of new products.
Understanding processes at an early stage
“We already offer our customers a wide range of services well in advance of production,” adds Dr. Marten Klukkert, Vice President Customer Development Center at Fette Compacting. “This means that we support pharmaceutical and nutraceutical companies from formulation development through to validation of their processes. The main aim is to integrate knowledge and technology to take the entire production process to a new level and ensure real added value for manufacturers.”
Comprehensive process consulting begins with a from many tests and applications: the QED (Qualified Expert Database) knowledge database provides empirical values from more than seven decades of tableting experience. This source makes it possible for us to find specific solutions for every product and every process. “With QED, we can offer our customers a comprehensive pool of specialist knowledge. This allows us to quickly determine the best processes and machine configurations,” enthuses Klukkert.

From left: Joachim Dittrich, CEO and Dr. Marten Klukkert, Vice President Customer Development Center at Fette Compacting.
Precise analysis of powders and tablets
While QED forms the digital basis for process partnerships, practical implementation begins in the laboratory area of Fette Compacting. This is where material and powder analysis is the first and already essential step in the development of medications and nutraceuticals. One of the core concerns of pharmaceutical production is the precise characterization and analysis of sensitive, highly regulated materials. To this end, Fette Compacting develops innovative solutions in its Customer Development Center that support users in the early development phase and create the best conditions for an efficient production process.
“The laboratory-based services start with advice on a new formulation and range from intensive powder characterization to test trials. In order to transfer the successes from the laboratory to the customer, we offer on-site training and application consulting,” reports Dr. Ina Petry, Group Lead Application Consulting at Fette Compacting. “More than ever before, consulting and training now go hand in hand with all aspects relating to the process. Manufacturers are often faced with the challenge that the pharmacists work on formulations of their active ingredients and excipients while the engineers work separately on production technologies. We can unite these two worlds by working closely together as an interdisciplinary team.”
With Lab Solutions, Fette Compacting offers a range of services that focus on the characterization of powders and tablets. “We help our customers to determine and analyze the critical material properties of their active ingredients, because the behavior of a powder is often the decisive factor for the efficiency and quality of subsequent tableting,” emphasizes Petry.
Fette Compacting’s Customer Development Center supports the principle of Quality by Design. This approach aims to optimize product quality through a deep understanding of production processes. “It means that we can identify the critical process parameters and quality attributes at an early stage and develop appropriate control strategies for, and with, our customers. In this way, they create robust processes for consistently high product quality,” Petry continues.
An essential part of this entails the analysis of the powder’s compaction and compacting properties. With the modular powder compaction analysis units of the F Lab Series, Fette Compacting can precisely examine the physical properties of powders. The analysis includes factors such as particle size distribution, density and flowability – all important parameters for a stable tableting process.

Dr. Ina Petry, Group Lead Application Consulting at Fette Compacting.
Emulating processes instead of simulating them
The next important step along the product life cycle involves process development. When passionate collaboration meets optimal technical equipment, entirely new possibilities open up. One of the most important technologies which will soon be in Schwarzenbek – and then in all Competence Centers – are several emulators that mimic real production conditions. In contrast to simulators, these analysis systems have the potential to test processes without conversion steps and loss of time under particularly close-to-production conditions. In the long term, they offer companies the advantage of validating their recipes and process parameters at an early stage and eliminating potential faults in the development phase before they occur in production. By using small quantities of material, valuable resources can be saved and precise predictions made about the subsequent production process.
Faster to production
Let’s move on to technology transfer: the transition from research and development to commercial production is often associated with considerable challenges. This is where Fette Compacting provides support in the form of a seamless transfer service. “We offer various solutions that enable our customers to transfer their projects to
production more quickly and react flexibly to changes,” explains Klukkert. Support during scale-up is particularly valuable for users, as this not only saves time but also reduces the risks that can occur during commercial production.
In addition to increasing efficiency and optimizing processes, sustainability is also playing an increasingly important role, as explained by Dr. Martin Sch?ler, Vice President Technology at Fette Compacting: “The concept of sustainability is deeply embedded in our DNA, in every development and design. We are highly committed to minimizing material consumption and making processes more resource-efficient. One key to this is the FE CPS continuous processing system, which enables us to work with minimal quantities of material during the development phase. This allows us to gain valuable insights with just a few kilograms of material, which considerably speeds up the development process and significantly reduces material loss.”

Dr. Martin Sch?ler, Vice President Technology at Fette Compacting.
FE CPS milestone
The FE CPS has also provided the decisive impetus to grow more strongly in the direction of process. As the system adapts to a wide range of production scenarios, it offers optimum conditions for development and production. With continuous direct pressing, the powder is fed from the dosing-mixing unit via the powder transport system into the tablet press without additional granulating. Compared to granulation-based production, several steps are eliminated, which reduces the space and energy requirements as well as the process costs.
The birthplace of this world first is Fette Compacting’s innovation center in Mechelen, Belgium. This is where the idea of revolutionizing tablet production through continuous manufacturing was born. By testing various material and process-related scenarios, the system was enabled to process a wide range of ingredients in a throughput range of five to over 200 kilograms per hour. It can dose and mix up to six different powdered raw materials and transfer them to the downstream processes.
In the meantime, numerous product trials and several sales have confirmed the enormous interest in the FE CPS on the part of pharmaceutical and nutraceutical producers. In addition, there is a key component that was developed in close cooperation between Schwarzenbek and Mechelen: embedded Process Analytical Technology (ePAT). “With ePAT, we can continuously monitor the most important quality in real time and react immediately in the event of deviations,” says Dr. Anna Novikova, Head of Process Consultancy at Fette Compacting. Using near-infrared spectroscopy (NIRS), ePAT monitors product quality directly during the production process. This technology makes it possible to check the chemical composition and physical properties of powders and tablets without incurring any interruptions or delays. “With ePAT, process control finally becomes simple, robust and efficient. The integrated sensor technology helps manufacturers ensure consistently high product quality and detect potential production errors at an early stage,” says Novikova.

Dr. Anna Novikova, Head of Process Consultancy at Fette Compacting.
Proven technologies and services
In addition, it goes without saying that batch-to-batch process technologies are still available to users. The decisive factor is which solution is best suited to a specific product and production environment. For classic tableting, for example, users can rely on the tablet presses from the new i Series. It offers modernized models that allow flexible adjustments, advanced energy management, high network capability, innovative safety solutions for operator protection, and many other design advantages.
The proven production support services and performance consulting also come into play, as Lars Plüschau, Vice President Global Sales at Fette Compacting, explains: “Customer support is crucial to the success of our customers. The comprehensive consulting, training and modernization services offered by Fette Compacting are aimed at optimizing production efficiency and product development time (time to market). Tableting tools also play an important role in this. By supplying machines and tools from a single source, interface problems can be avoided, which considerably simplifies the production process and saves customers both time and money. This underlines our orientation as a reliable process partner that not only offers machines, but also customized solutions for the entire life cycle of a product.”

Lars Plüschau, Vice President Global Sales at Fette Compacting.
The journey continues
“Together – from lab to production” is the direction for Fette Compacting’s development journey. Meanwhile, the Schwarzenbek site is already working on its next innovation: expanding cleanroom capacities with the new ISO 8 standard opens up additional opportunities to test highly active substances under strictly controlled conditions. The planned cleanrooms will expand the portfolio, as more products can be tested under real conditions using all of the technologies available at Fette Compacting. This will make it possible to further expand collaboration with customers from the early stages of development, ultimately shortening the time to market.